Introduction
Batteries that are rechargeable provide energy to many of your daily, everyday devices. From a camera to a hybrid car you use rechargeable batteries in each device that you own. The utmost common rechargeable battery used by consumers, is called Nickl Metal Hydride (NiMH). NiMH batteries are known for their versatility, and are a dependable power source. A standard NiMH cell consists, of a positive electrode made with nickel and a negative electrode made up of an alloy that absorbs hydrogen, with a potassium hydroxide (KOH) electrolyte that separates these two electrodes. Each cell produces approximately 1.2 volts, which is standard; thus, users will typically use multiple cells connected together in either series or parallel to increase the total voltage or capacity of the battery as needed. Over time, NiMH technology has taken over many areas where Nickel-Cadmium (NiCd) batteries were previously the most dominant type of rechargeable battery because NiMH technology offers greater energy density than NiCd and does not contain toxic cadmium. Because of this, NiMH batteries offer cleaner energy and longer life for many types of consumer electronics.
Principle and Structure
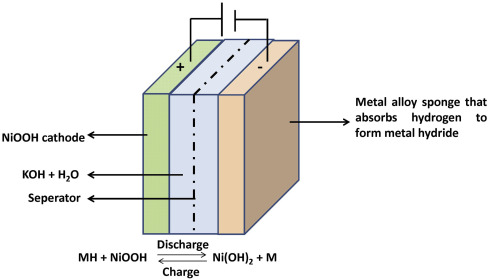
Figure 1 shows the simple structure of a NiMH cell and showed the arrangement of the positive electrode, negative electrode, separator, and electrolyte in a cylindrical arrangement. This graphic design helps clarified how the electrochemical apparatuses function together as a sealed rechargeable system.
The functioning principle of a NiMH battery relies on reversible electrochemical reactions, between the nickel-hydroxide positive cathode and the metal-hydride negative anode. Upon discharge the nickel oxyhydroxide (NiOOH-type) positive electrode is reduced to nickel hydroxide (Ni(OH)₂) and the metal hydride releases neutral hydrogen atoms that are oxidised to electrons. Those electrons then flow through the external circuit to supply energy to the device. And hydroxide ions (OH⁻) from the alkaline electrolyte maintain the charge separation of the electrodes. During charging, these reactions reverse: electrical energy drives the formation of the higher-oxidation nickel oxyhydroxide phase at the cathode while hydrogen is re-absorbed into the alloy at the anode. This reversible process allows NiMH batteries to maintain a stable voltage profile across many charge–discharge cycles.
Figure 1: Basic Structure of a NiMH Battery
Advantages and Limitations
NiMH batteries have numerous benefits, with higher energy density associated to NiCd batteries, decent power output for high-drain devices, ecological safety, and long-standing reusability. Though, they also have limitations such as self-discharge over time, effectiveness losses throughout fast charging, and more complex charging requirements. Different battery chemistries; NiMH batteries do not exhibit, a clear voltage plateau when fully charged; therefore, smart charging techniques, are needed to prevent overcharging and overheating.
Table 1: Comparison of NiMH Advantages and Limitations
| Feature | Advantages | Limitations |
| Energy Density | Higher than NiCd, moderate | Lower than Li-ion |
| Environmental Impact | No toxic cadmium | Requires proper disposal |
| Charge/Discharge | Reusable, handles high current | Self-discharge, efficiency loss at fast charging |
| Charging Method | Can use CC or trickle | Needs –ΔV or temperature detection for full charge |
Charging Methods and Best Practices
The charging of NiMH batteries must be controlled tightly to ensure longevity and safe operation. They can’t just be charged with simple constant-voltage chargers like disposables cells. Dedicated NiMH chargers monitor the charging process and take into account various signals, including the voltage behavior or temperature increase, or elapsed time, to estimate the charge level and end of the process. A well-known procedure in this area is the negative delta-V (–ΔV) detection, in which the charger detects a minute drop in voltage right after the cell attains its full capacity. Temperature related methods (ΔT or ΔT/dt) are also widely applied since NiMH cells have a significant increase in temperature at near full charge. Some chargers use trickle charging, a small, continuous charge to maintain near full charge and compensate for natural self discharge, or negative charging, which reverses some loss charge from discharging at a high rate.
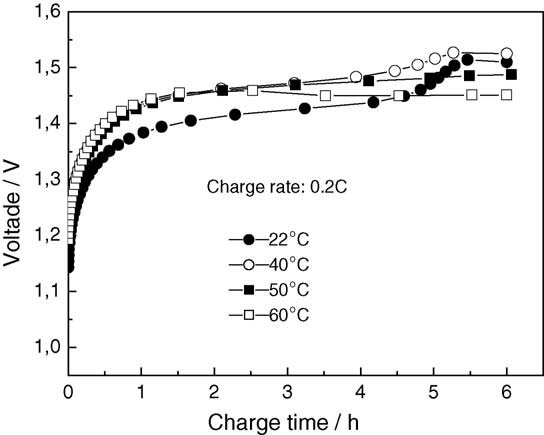
These effects can be seen in Figure 2, where we display a typical NiMH charge curve. The Figure illustrates the progressive increase in voltage during constant-current charging, the small voltage depression at full charge (–ΔV) and the simultaneous temperature increase. This visual profile provides a good explanation of why the sophisticated chargers now available utilize several detection methods to prevent overcharging, overheating and premature aging.
Figure 2: NiMH Charging Curve
In the interest of safe charging, Table 2 summarizes common charge termination methods with their advantages and disadvantages. These are –ΔV detection, temperature-sensing control, timer-based cutoffs, and trickle-maintenance modes, each applicable to different charge rates and battery sizes.
Table 2: Key charging methods for NiMH batteries
| Charging Method | Description | Pros | Cons |
| ΔV Detection | Stops charging when voltage peaks then drops slightly | Accurate full charge | Sensitive to temperature variations |
| Temperature Sensing (ΔT) | Stops charging when temperature rises sharply near full charge | Prevents overheating | Requires precise sensors |
| Timer-Based | Stops charging after a preset time | Simple implementation | Risk of under/overcharging |
| Trickle / Maintenance | Applies small current to offset self-discharge | Keeps battery ready | Not suitable for fast charging |
Applications of NiMH Batteries
NiMH batteries are commonly used, in domestic electronics for example AA/AAA rechargeable cells for cameras, remote controls, toys, and flashlights. They are also working in high-drain devices, that require bursts of current, backup emergency systems, and historically in hybrid electric vehicles. Their balance of capacity, reliability, and eco-friendliness makes them suitable for many portable and stationary power applications.
Conclusion
NiMH batteries offer a reliable and environmentally conscious choice, for rechargeable power. Understand their chemical structure charging behavior, and practical limitations is essential for maximizing efficiency and longevity. Proper charging techniques including, monitoring voltage temperature, and time ensure safe operation and help prevent overcharging, making NiMH batteries a versatile solution across a wide range of applications. For more information about battery technologies and related solutions, you can also visit Sinexcel-RE.
FAQ
- What is a NiMH battery?
A rechargeable battery using nickel and metal-hydride chemistry, offering higher energy than NiCd. - How does it work?
Stores hydrogen in the anode during charging; releases it as electricity when discharging. - Why do NiMH batteries self-discharge?
They naturally lose charge over time; low-self-discharge (LSD) types reduce this. - How should I charge a NiMH battery?
Use a smart charger with –ΔV or temperature cutoff to prevent overcharging. - What is negative Delta-V (–ΔV)?
A small voltage drop signaling the battery is full; chargers stop charging at this point. - Can NiMH replace alkaline batteries?
Yes, especially in high-drain devices. They are reusable and stable. - Are NiMH batteries safe?
Yes, if used with the correct charger and not overcharged. - Where are they used?
AA/AAA cells, cameras, remotes, emergency systems, and hybrid vehicles.